EXECUTIVE ABSTRACT
Strength of recommendation: Weak for using the technology
Technology: Sorafenib
Indication: Sorafenib is an antineoplastic agent indicated for the treatment of unresectable hepatocellular carcinoma (which can´t be treated with surgery) and renal carcinoma in an advanced stage.
Characterization of the technology: Sorafenib blocks intracellular kinases and cell surface, inhibiting the growth of cancer cells.
Question: Is sorafenib effective and safe for the treatment of patients with malignant neoplasms of liver?
Search and analysis of scientific evidence: We searched the databases The Cochrane Library (via BIREME), Medline (via Pubmed), Lilacs, Centre for Reviews and Dissemination (CRD) and Tripdatabase aiming to find systematic reviews (SR) of clinical trials comparing sorafenib with other therapeutic options for the treatment of malignant neoplasm of liver. We also selected health technology assessments (HTA) on sites of international agencies and REBRATS. We searched complementary for clinical trials about cholangiocarcinoma, angiosarcoma e hepatoblastoma in Medline (via Pubmed). Quality of the evidence and strength of recommendation were evaluated using the GRADE system.
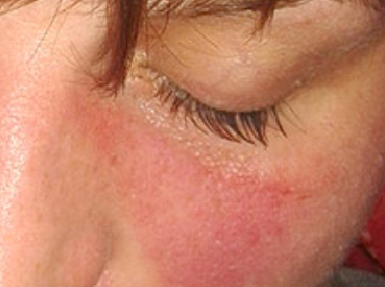
Summary of results of selected studies: 11 studies were included, seven SR and four HTA. The reviews show that sorafenib is effective compared with the control group for outcomes of survival and time to tumor progress. Patients who received sorafenib had a higher risk of suffering major adverse events such as thromboembolic and bleeding events, hypertension and dermatologic toxicity. Two SR presented high quality of evidence, while the rest, moderate quality. All reviews showed weak recommendation for using sorafenib. We included two Phase II clinical trials about cholangiocarcinoma and angiosarcoma. We didn’t found any clinical trials about hepatoblastoma.
Recommendations: The Diagnostic and Therapeutic Guidelines for Liver Cancer in Adult from the Ministry of Health include sorafenib as a therapeutic alternative among other antineoplastic agents, but specific recommendations on its indication is not stated. The strength of recommendation is weak for using sorafenib in the treatment of advanced hepatocellular carcinoma based on available evidence of effectiveness. Furthermore, this drug is associated with serious adverse events and this should be carefully considered in the decision to initiate therapy, assessing the patient’s condition and monitoring for early detection of these events. The increased survival caused by the use of sorafenib is statistically significant, but the difference compared to the control group is modest and based on insufficient evidence, therefore with little clinical value. Furthermore, treatment with sorafenib has high cost and therefore the use of this drug to ensure to the patient an increase in the survival of a few months should be collated considering the health demands and priorities in a context of limited resources.
Full content in Portuguese















Adicionar Comentário