DOI: 10.13140/RG.2.1.2101.9040
ABSTRACT
Intensity of recommendations: Weak against of technology
Technology: Sunitinib
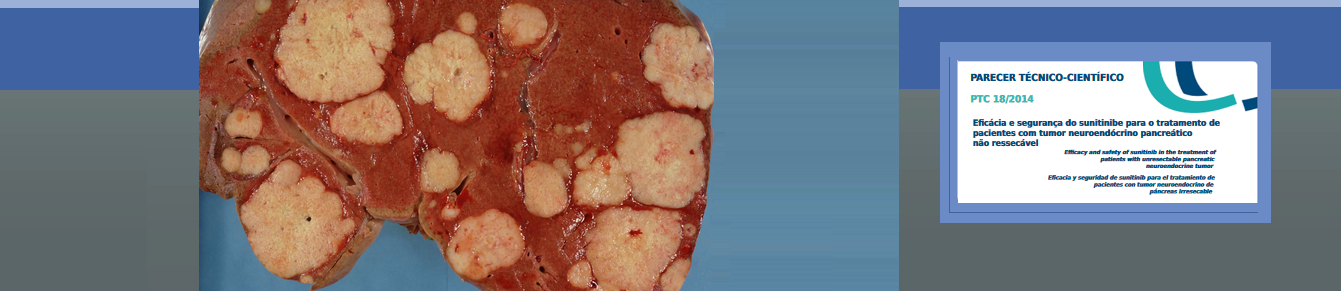
Characterization of technology: Sunitinib is an antineoplastic, inhibitor of protein kinase inducing to malignant cell to enter apoptosis and reducing proliferation. It is indicated for the treatment of renal cell carcinoma, gastrointestinal stromal tumors after disease progression or in case of no tolerance to imatinib, and unresectable Pancreatic Neuroendocrine Tumor PNET (those that cannot be operated).
Question: Is sunitinib effective and safe for treating patients with unresectable PNET?
Search and analysis of scientific evidence: the databases The Cochrane Library (via BIREME), Medline (via Pubmed), Lilacs and Centre for Reviews and Dissemination (CRD) were searched. Manual search was also performed the references of the studies found. A search of Health Technology Assessments (HTA) was held in the Brazilian Network for Technology Assessment and Health (REBRATS) and international agencies as agencies y units of Evaluación de Tecnologías Sanitarias (AUnETS/Spain), Canadian Agency for Drugs sites and Technologies in Health (CADTH/Canada), National Institute for Health and Clinical Excellence (NICE/UK), Health Technology Assessment Programme (NIHR/UK) and Pharmaceutical Benefits Advisory Committee (PBAC/ Australia). Eligible cohort studies and clinical trials, systematic reviews and economic evaluations conducted in any geographic region, with patients of both sexes aged over 18 years, with unresectable PNET were considered. Studies comparing the use of sunitinib any other therapeutic intervention, placebo or no treatment intervention groups were selected. Outcomes were considered as progression-free survival, overall survival, time to response, objective response rate, duration of response and adverse events.
Summary of results of selected studies: After thorough reading, five studies were selected: one phase III clinical trial, two phase II clinical trials, a matching-adjusted indirect comparison of two phase III clinical trials and a cost-effectiveness analysis. Compared with placebo, the use of this drug provided improved overall survival, progression-free survival and response to treatment. Compared to everolimus, no significant differences were observed in effectiveness. However, neutropenia and hypertension were significantly associated with sunitinib compared with the everolimus. Furthermore, the ratio of treatments cost and clinical outcomes tends to discourage sunitinib.
Recommendations: Considering the available evidence and its methodological quality, the strength of the recommendation is weak against the use of sunitinib as first choice for treatment of patients with non-resectable PNET. Their use is only recommended in cases of everolimus contraindication.
Full content in Portuguese














Adicionar Comentário