DOI: 10.13140/2.1.2713.4244
EXECUTIVE ABSTRACT
Strenght of recommendation: Weak against the technology.
Technology: Sorafenib. Indication: Sorafenib is an antineoplastic agent indicated for the treatment of renal carcinoma and hepatocellular carcinoma, both in advanced stage.
Characterization of the technology: Sorafenib blocks intracellular kinases and cell surface, inhibiting the growth of cancer cells.
Question: Is sorafenib effective and safe for the treatment of patients with malignant kidney neoplasms?
Search and analysis of scientific evidence: We searched the databases The Cochrane Library (via BIREME), Medline (via Pubmed), Lilacs, Centre for Reviews and Dissemination (CRD) and Tripdatabase. We sought systematic reviews (SR) of clinical trials comparing sorafenib with other therapeutic options for the treatment of malignant neoplasm of kidney. We also selected health technology assessments (HTA) on sites of international agencies and Brazilian Health Technology Analysis (REBRATS). We selected studies published in English, Portuguese or Spanish.
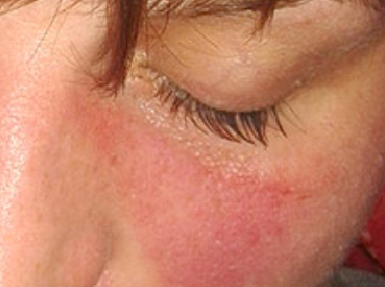
Summary of results of selected studies: Nine studies were included, eight SR and one HTA. The reviews show that sorafenib for outcomes of survival is more effective that placebo, however when this medicine is compare which others therapeutic alternatives, he is equally effective that interferon, and less effective that sunitinib. Patients who received sorafenib had a higher risk of suffering major adverse events such as thromboembolic and bleeding events, hypertension and dermatologic toxicity. Three SR presented moderate quality of evidence, while the rest high quality. All reviews showed weak strength of recommendation, one study for using sorafenib compared to placebo and the rest against intervention.
Recommendations: The strength of recommendation is weak against sorafenib in the treatment of metastatic renal carcinoma based on available evidence of effectiveness and safe. The use of drugs inhibiting vascular endothelial growth factor (anti-VEGF) is associated with serious adverse events. The increased survival caused by the use of drugs anti-VEGF is modest and based on insufficient evidence, therefore with little clinical value. Furthermore, treatment with anti-VEGF drugs has high cost. Therefore we recommend the use of these drugs only when surgical alternatives aren’t available.
Full content in Portuguese















Adicionar Comentário